1. Overview
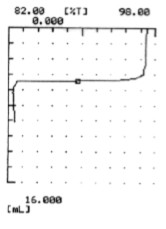
Total hardness of tap water means calcium carbonate in mg/L equivalent to calcium ion and magnesium ion in water. According to JIS K 0101-1998, ASTM D 1126-96 and ISO6059:1984, it is measured by chelatometric titration with EDTA 2Na solution. The automatic potentiometric titration goes on using the photo sensor (Photometric titration) to the endpoint.
The titration volume is converted to calcium carbonate to obtain total hardness. The endpoint is determined by the color change of the EBT (Eriochromeblack T) indicator from red to blue.
2. Equipment
a. Automatic potentiometric titrator (preamplifier PTA)
b. Photometric sensor+ Interference filter (630nm)
3. Reagents
Titrant: 0.01mo1/L EDTA2Na solution
Additive: 100g/L potassium cyanide solution, 100g/L hydroxylammonium chloride solution, Ammonium chloride -Ammonia buffer (pH10)
Indicator: Eriochromeblack T