This application note helps you to get started with spectroelectrochemistry and clearly shows what components you need to successfully record a UV-VIS spectrum during CV.
The experiment in this application note shows that a change in potential causes reduction and oxidation of methyl viologen not only by measuring the electric current but also by measuring the change in the transmittance and absorbance of the solution in a cell.
Potentiostat:
An electronic device that controls the potential (or voltage) difference between two electrodes and measures the current between them is called a potentiostat. A three-electrode setup, comprising a working electrode, reference electrode, and counter electrode, is very common. The potential is applied between the working electrode and the reference electrode, while the current is measured between the working electrode and counter electrode. This way the potential of the working electrode is known, while a current is flowing.
The electrodes can be very small like micro-electrodes in a conductive solution or large coated metal compounds in an acidic environment.
Spectrometer:
Optical spectroscopy is a technique that is used to measure light intensity in the ultraviolet (UV), visible (VIS), near-infrared (NIR), and infrared (IR) range of the electromagnetic spectrum. Spectroscopic measurements are used in many different applications, such as color measurement, characterization, or concentration determination of chemical components.
Reagents:
• 0.1 M KCl
• 1 mmol/L methyl viologen (MV2+) in 0.1 M KCl
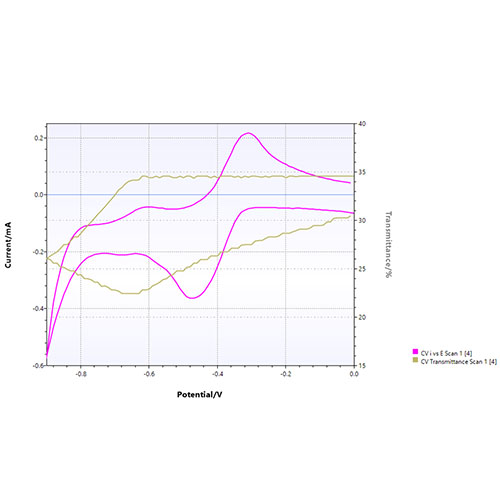
Results:
A decrease of transmittance is visible starting at around -700 mV, which is a result of the MV 2+ reduction to MV+ and is also visible in the CV as a small peak. From -0.7 V to 0 V the transmittance increases again because MV+ is oxidized. Combining electrochemistry and spectroelectrochemistry results in more insights and a better understanding into the oxidation and reduction processes.