Pack of 10 x 10 ml syringes for use with the IP 570 Proficiency Test Scheme (SA4032-0).
IP 570 PTS Quarterly Membership – SA4032-0
Support analytical competency and compliance with ISO 17025 recommended practice in IP 570/14a.
Validation of apparatus performance is stated as a mandatory requirement at least every three months in IP 570/14a. Participation in the Seta IP 570 Proficiency Testing Scheme is the preferred means to achieve this requirement, enabling laboratories to evaluate their performance for testing H2S in the liquid phase using the Seta H2S Analyser.
Participants in the statistical quality assurance programme can continually monitor and compare their performance by analysing their data against similar laboratories in accordance with ISO/IEC 17025. This gives the ability to demonstrate compliance with laboratory accreditation requirements, identify any potential equipment or operational bias and provide added confidence to staff and customers. Ultimately helping to increase laboratory efficiency whilst reducing costs.
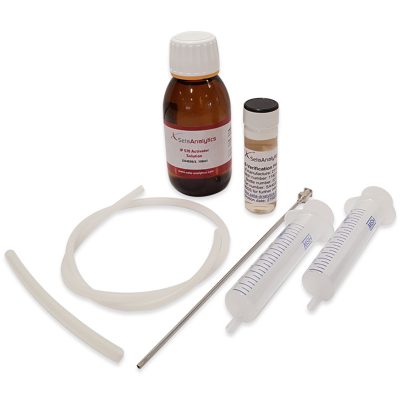

IP 570 PTS Quarterly Membership – SA4032-0
Features
- Ability to demonstrate compliance with laboratory accreditation requirements (ISO 17025)
- World-wide inter-laboratory comparison of test results and performance
- Identify any potential equipment or operational bias
- Provides added confidence to laboratory staff and customers
- Reduced potential for dispute
specifications
| CCCN CODE | Tariff 90269000 |
|---|
Manufacturers
show DATA — DO NOT CHANGE ID–
Industries
show DATA — DO NOT CHANGE ID–
Methods
show DATA — DO NOT CHANGE ID–
Spares and accessories for SA4032-0
10 ml Syringe (pack of 10) – SA4030-003
Needles (pack of 10) – SA4030-001
20 ml Syringe (pack of 10) – SA4030-004






