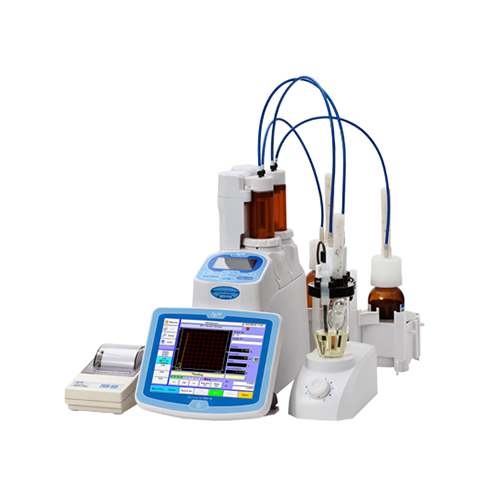
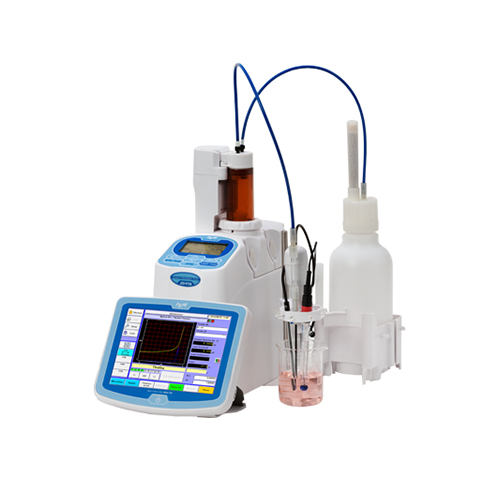
.
Titration
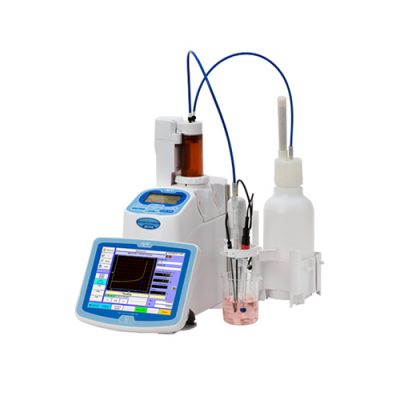
Titration, a crucial quantitative measurement in analytical chemistry, involves the complete reaction of an analyte with a reagent. It’s a powerful tool used to determine the amount or concentration of a substance. Modern titrators, equipped with end-point indication, accurately doses the titrant and uses potentiometric electrodes to determine the endpoint electrochemically. This advancement has made potentiometric titrations increasingly popular.